Describe the Electrolysis of Copper Sulphate Solution Using Copper Electrodes
It was found that after electrolysis the absorbance of the solution was reduced to 50 of its original value. Turn on power supply.

Question Video Writing The Equation For The Reaction At The Anode During The Electrolysis Of Copper Sulfate Solution Using Inert Electrodes Nagwa
Switch off the circuits and remove the cathodes.

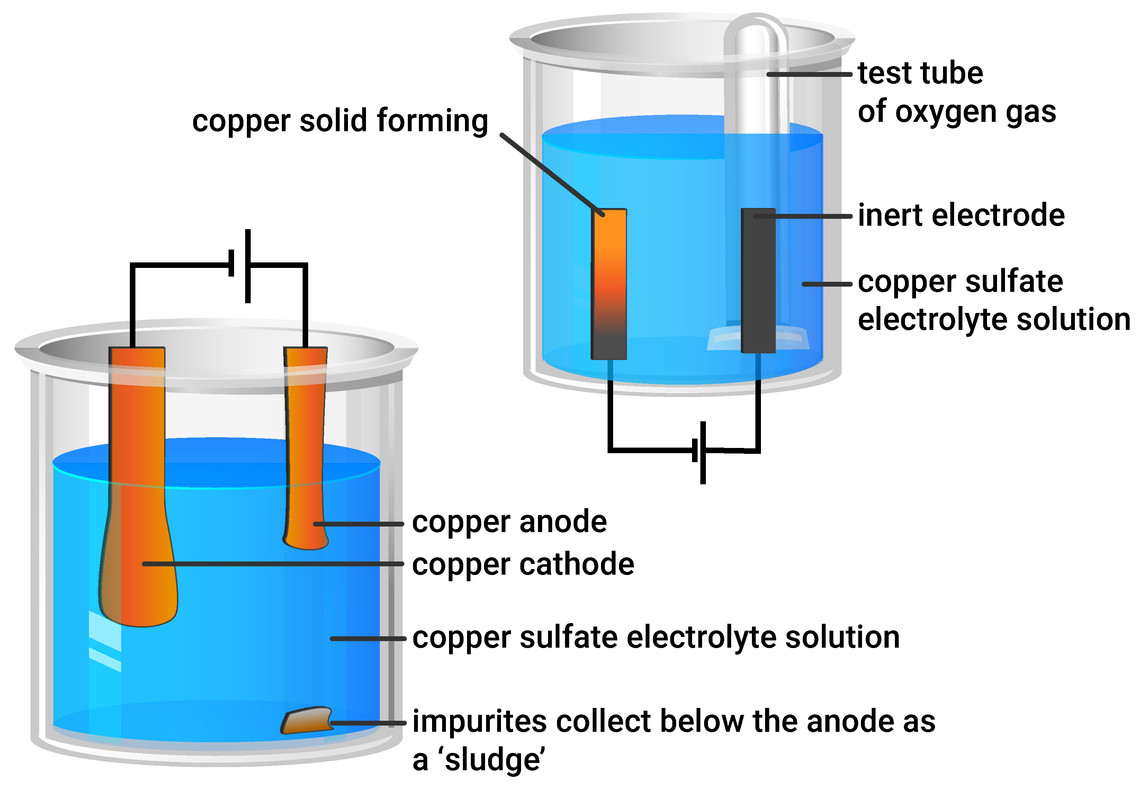
. Whenever copper sulfate or CuSO 4 is added to water it gets dissolved in the water. Fill a small test tube with copper sulfate solution and position over the positive. Bubbles of gas oxygen are formed at the anode.
There are two parts to the core practical electrolysis of copper sulfate solution first using copper electrodes and second using inert graphite electrodes. What does the electrical supply do during the electrolysis of copper sulphate using non-inert electrodes. CuSO 4 Cu 2 SO 4 2-Copper sulphate Cation Anion.
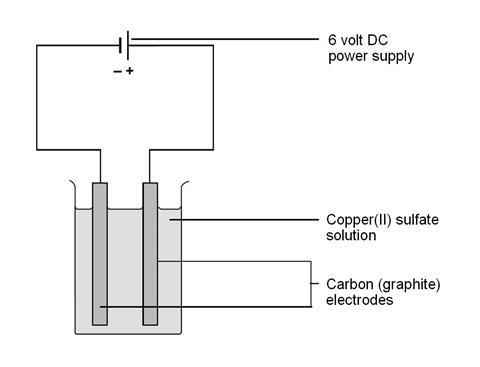
Instead copper atoms in the electrode release electrons and go into solution as Cu 2 aq ions. Place two graphite rods into the copper sulfate solution - attaching one electrode to the negative terminal of a dc supply and the other electrode to the positive terminal. Tap card to see definition.
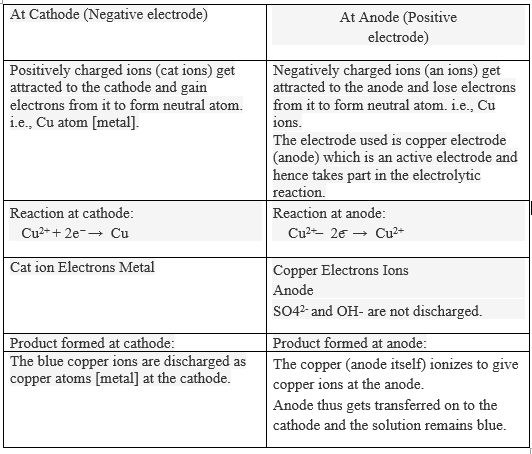
At the negative electrode copper ions gain electrons and form copper atoms. A half-equation shows what happens at one of the electrodes during electrolysis. Electrons are shown as e-.
They continue towards the copper electrode. The use of copper electrodes illustrates how copper is refined industrially. Set up an electrolysis cell using graphite rods as.
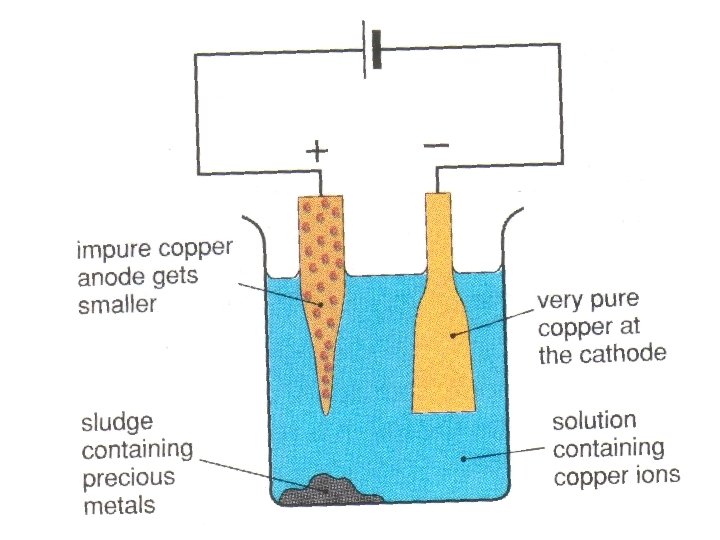
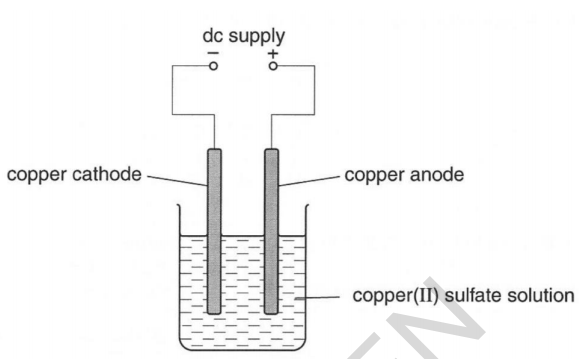
Electrolysis of i copper sulfate solution with copper electrodes and ii acidified water with inert electrodes. When a solution of copperII sulfate is electrolysed using graphite electrodes copper metal is formed on the cathode by reduction of copper ions in the sol. This process is used industrially to purify copper.
Copper electrodes Pour copper sulfate solution into a beaker. Cus Cu 2 aq 2e-The net effect. In the case of electrodes with an immersed area of 8 x 5 cm currents of 1 A in one case and 05 A in the other two cases would be very suitable.
Set up an electrolysis cell using graphite rods as electrodes and copperII sulphate solution as electrolyte. 3 rows The electrolysis of copper sulfate solution using copper electrodes. Electrolysis of copper sulfate solution using non-inert copper electrodes.
Mass of copper deposited from copper II sulphate solution using a current of 02 A 005 over 300 seconds. Pour some copper sulfate solution into a beaker. For every copperII ion discharged at the cathode a new one is released into solution at the anode.
Instead of using inert electrodes chemists tried placing pure copper electrodes in copper sulphate solution. I 020 A 005 t 300 s 001 Q 020 x 300 600 C moles of electrons. Calculate the concentration of copper sulphate in the solution to begin with.
Electrolysis of Copper sulphate solution. Ne Q F. Q I x t.
Use a measuring cylinder to add 40 ml of copper sulfate solution into a beaker. Describe the method of the electrolysis of copper sulfate solution using copper electrodes. A constant current of 2 mA was passed for 193 min.
4OH-aq O 2 g 2H 2 Ol 4e-At the cathode. Discharge of Ions at Electrodes. It pulls electrons off copper atoms at the anode and it offers electrons at the cathode to nearby Cu2 ions.
They found that the copper on the cathode became bigger and the copper on the anode became smaller. The electrons generated by the zinc anode travel through the outer wire and record a reading on the voltmeter. That means that how much the anode has lost the cathode should have gained.
In the electrolysis of cupper ii electrodes and copper sulphate solution the copper ions at the anode give ions to the cathode. This means that the solution must be electrolyte. Copper sulphate solution 250 ml was electrolysed using a platinum anode and a copper cathode.
The copperII sulfate solution is unchanged. This is a method. All that happens is a transfer of copper from the anode to the cathode.
Part 1 - Investigation using inert electrodes. The use of copper electrodes illustrates how copper is refined industrially. Dilute copper sulphate solution.
These are the half-equations. This experiment is designed to demonstrate the different products obtained when the electrolysis of copperII sulfate solution is carried out first with inert graphite electrodes and then with copper electrodes. Electrons enter the copper electrode where they combine with copper ions II in the solution reducing them to copper metal.
Pour some copper sulfate solution into a beaker. Electrolysis of Copper Sulfate. Q 600 C F 965 x 10³ C mol-1 ne 600 965 x 10³ 622 x 10-4 mol.
You may wish to split this over two or more lessons depending on the length of your lessons. Now we will immerse two copper electrodes in that solution. Copper electrodes are active electrodes.
Example 2 - Electrorefining of copper. Measure and record the mass of a piece of copper foil. So the chemists then tried putting impure copper at the anode copper from smelting.
As CuSO 4 is an electrolyte it splits into Cu cation and SO 4 anion ions and move freely in the solution. The copper anode has reduced in mass following electrolysis whilst the copper c. Cu Cu 2 2e-oxidation.
A deposit of copper forms on the cathode. Social and Applied Aspects Use of scrap iron to extract copper. Click card to see definition.
Wash dry and then lightly clean them with emery cloth or paper. Measure and record mass of copper foil and attach to positive terminal one to the negative terminal. The electrode to which the reduction occurs is called cathode.
During the electrolysis of copper sulphate aqueous solution using copper electrode the reaction taking place at the cathode is C u 2 a q 2 e C u s C u I I ions having lower discharge potential than proton so will be liberated at the cathode in preference to protons. This corresponds to current densities of about 0025 A per cm2 and 0012 A per cm2. Method 1 involves the electrolysis using copper electrodes and is quantitative as students measure the mass change of the.
Attach it to the negative terminal of a DC supply and dip the copper foil into the copper sulfate solution Repeat with another piece of copper foil but this time attach it to the positive terminal Make sure the electrodes do not touch each other then turn. The anode is positively charged ions and the cathode are negatively charged ions.
From The Electrolysis Of Concentrated Copper Ii Sulphate Using Carbon Electrodes What Product Will I Get Socratic

Principle Of Electrolysis Of Copper Sulfate Electrolyte Electrical4u
Explain Electrolysis Of Copper Sulphate Solution With Diagram Chemistry Topperlearning Com C2b462huu

The Electrolysis Of Copper Sulphate Solution Using Copper Electrodes Gcse Science Marked By Teachers Com

Electrolysis Ppt Video Online Download
In The Electrolysis Of Cuso4 Using Copper Cathode And Graphite Anode What Will Happen To The Colour Of The Solution Quora
Why Does Copper Sulfate Fade During Electrolysis Quora
Definitions Of Terms Uses Of Electrolysis Learn To

Y12 Electrolysis Aqueous Copper Ii Sulphate Using Copper Electrodes Youtube

Principle Of Electrolysis Of Copper Sulfate Electrolyte Electrical4u
Electrolysis Of Copper Ii Sulfate Solution Experiment Rsc Education
Explain Electrolysis Of Copper Sulphate Solution With Diagram Cbse Class 8 Learn Cbse Forum
Copper Plating Electrolysis Factory Sale 56 Off Floresdekiskeya Org
Electrolysis Of Copper Sulfate Solution Edexcel Core Practical Revisechemistry Uk

Icse Chemistry Notes Chapter Wise Practice Papers Electrolysis Of Copper Sulphate Using Copper Electrodes

Electroplating Formula Chemistry Clearance 60 Off Floresdekiskeya Org

New Gcse Separate Sciences Ocr Gateway Sb Page 144
Electrolysis Of Copper Ii Sulfate Solution Experiment Rsc Education
Comments
Post a Comment